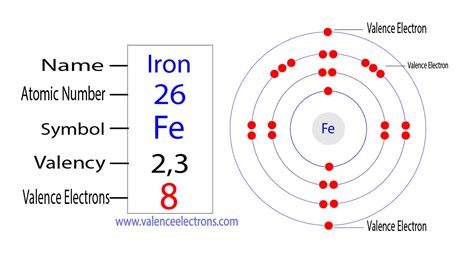
valence electrons in iron|valence electrons chart : Tuguegarao The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence electrons Corinna Kopf Threatens Fans With Lawsuit After OnlyFans Photos Leak Read more Last week, Kopf took to Twitter to announce that she would begin an OnlyFans profile in exchange for a 500,000 likes .
PH0 · valency of iron
PH1 · valence electrons chart
PH2 · number of valence electrons list
PH3 · list of valence electrons for each element
PH4 · iron valence electrons count
PH5 · iron valence elc
PH6 · how to calculate valence electrons
PH7 · copper valence electrons number
PH8 · Iba pa
Be at your most productive and stay connected with Outlook. Send, receive, and manage your email, and use the built-in calendar to keep track of appointments and events.
valence electrons in iron*******You may assume that the valences of the elements—the number of electrons .
The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence electrons Learn how to write the complete electron configuration of iron in two ways: through orbitals (Bohr model) and through orbitals (Aufbau model). See the electron . To find the number of valence electrons for Fe (Iron) we need to look at its electron configuration. This is necessary because Fe is a transition metal (d b.As previously stated, iron has two valence states: +3 and +2. As a result, when it gives up the two 4s electrons, it gains a valency of +2. Iron can also give up either of the paired . Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical . The valence electrons are be the 4s and 4p electrons. Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [Ar]4s 2 3d 6 .
Science. Iron has 8 valence electrons. Since iron is a transition metal, it can use electrons in its d subshells as valence electrons. A transition metal’s valence electrons are electrons that . Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. There are two electrons in the 4s subshell and 6 electrons in the 3d subshell, .The electrons which are distributed in the outermost shell of the atom are called valence electrons. These valence electrons can form a chemical bond only if the outer shell remains unclosed. Valence electrons in an Iron(Fe atom: The element Iron Fe is in the fourth period and belongs to the d-block (group 8). The atomic number of Iron Fe is 26.An element’s valency is the number of electrons it receives, gives away, or shares in order to achieve a noble gas or stable configuration. In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. As previously stated, iron has two valence states: +3 and +2.
Electronic Configuration of Iron. The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a transition metal from group 8 of the periodic table’s first transition series. It is the most abundant element on Earth by mass, coming in second to oxygen (32.1% and 30.1%, respectively), and it makes up much .
The number of valence electrons in an iron atom (Fe) is 8. Iron has 8 total valence electrons. Valence electrons are the outermost shell electrons that are responsible for determining how the atom will react with other atoms and hence, their chemical properties. Iron (Fe) is a transition metal located in group 8 and period 4 of the .
Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Iron is 26. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. Hint To find the number of valence electrons in iron atom , you should first know that how many electrons are present in iron and then, calculate the total number of electrons present in the s, p and d-orbitals to get the valence electrons. Now you can easily answer the statement. Complete answer:-Iron is a metal which comes under the .
Iron Valency & Electron Configuration. Iron (Fe) is a transitional metal that’s valency depends upon its oxidation state. When it combines with the sulfate radical to form ferrous sulfate, FeSO 4, its valency must be +2, because the valency of the sulfate radical, as determined from the bond it forms with hydrogen, is -2.
An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .valence electrons in ironAn atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can . As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into .valence electrons in iron valence electrons chart Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom. The electron configuration of a oxygen atom is. O: 1s22s22p4 (1.9B.1) (1.9B.1) O: 1 s 2 2 s 2 2 p 4. which may be shorted.Iron is an essential element for all forms of life and is non-toxic. The average human contains about 4 grams of iron. A lot of this is in haemoglobin, in the blood. Haemoglobin carries oxygen from our lungs to the cells, where it is needed for tissue respiration. Humans need 10–18 milligrams of iron each day.
Example \(\PageIndex{1}\): Valence Electrons in Transition Metals. Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements. . The carbon is converted into CO, which is the reducing agent that accepts electrons so that iron(III) can be reduced to iron(0).The $$4s^23d^6$$ electrons. Iron thus has 8 valence electrons! Was this answer helpful? 1. Similar Questions. Q1. How many valence electrons does iron have? View Solution. Q2. How Many Valence Electrons Does Helium Have? View Solution. Solve.
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second .In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can .
Similarly, the observed electron configuration of copper is [Ar] 4s 1 3d 10 instead of [Ar] s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .
valence electrons in iron|valence electrons chart